Recycling of Photovoltaic (PV) Batteries
Overview
Batteries are used whenever electrical energy is needed, but there is neither a direct connection to the public electricity grid nor a generator-based stand-alone supply. Batteries store electrical energy as chemical energy. During discharge, the chemical energy is re-converted into electrical energy. Depending on the battery system, this process is either irreversible or reversible.
There are two types of batteries:
- primary batteries
- secondary batteries
Lead-acid batteries are called secondary batteries or accumulators since they are rechargeable. They again can be divided into starter and industrial batteries. Starter and industrial batteries are used to provide large quantities of energy (e.g. to start a car, operate electric vehicles, as energy storage medium for solar applications, as short-term emergency power source, etc.). Units generally weigh from a few kilograms to one ton.
In the lead-acid battery sector, starter batteries have by far the largest share. In 1995, approx. 96 million units were produced worldwide[1]. An annual production growth rate of 2% is expected. Especially in developing countries, where the number of cars is growing over-proportionately, high growth rates in the use of lead-acid batteries are to be expected. Studies carried out in Botswana indicate that the number of batteries used in the automobile sector will grow by 40-50% over the period from 1995 to 2005[2]. If we consider China alone, the most populous country of the world, which currently starts to introduce private car transport, it is obvious that high growth rates in the consumption of starter batteries must be expected in the future, especially in developing countries.
Returning used lead batteries to the recycling loop has a long tradition. Thanks to the compactness of a battery, its high lead proportion (95%) and relatively high metal prices, it has been worthwhile for consumers to return their own or collected car batteries to the scrap trade or secondary smelters. The return rate of spent batteries was thus already high in times when catchwords such as resource conservation and environmental protection, recycling, closed-loop materials management etc. did not yet play a role. Even today, the success of lead battery recycling in developing countries continues to be determined largely by the potential earnings of scrap collectors and traders. In industrialised countries, statutory requirements to take back spent batteries have compensated for the loss of economic incentives in spent battery return.
In most European countries, battery retailers are under obligation to take back spent batteries. Lead batteries also come from repair workshops, the reprocessing of scrap car bodies and at municipal collection centres. In Germany, for example, this well functioning and effective collection system has led to a return rate of more than 95% for starter batteries and almost 100% for industrial batteries. In developing countries, too, return rates of up to 80% can be achieved where buyingup structures for spent batteries are in place. In Zimbabwe[3] for example, the entire demand for local battery production is covered by recycling of used batteries.
Battery Scrap – Raw Material for Recycling
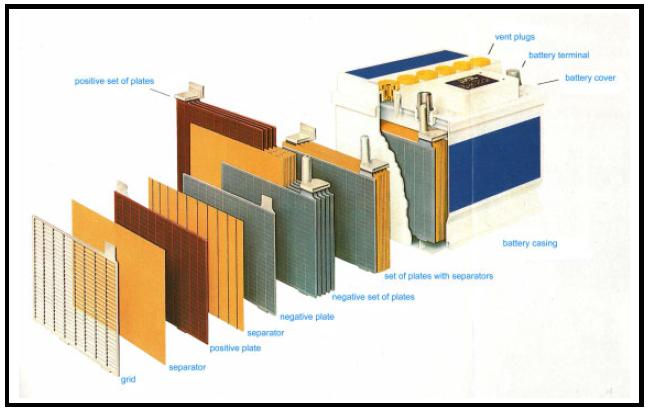
The major source of raw material for lead recycling are starter batteries from motor vehicles. Modern car batteries consist of a PP (polypropylen)-casing, plates (grids and paste), connectors/poles and bridges, and PP-separators as insulators between the plates (Fig 1).
Paste consists of Pb, PbO reactions which take place during charge and discharge of a lead acid battery are:
- charging: 2PbSO and 2PbSO4. The electro-chemical4+ 2H2O→ PbO2+Pb + H2SO4
- discharging: PbO2 + Pb + H2SO4 → 2PbSO4 + 2H2O
Therefore, recyclers have to be aware that batteries in hard rubber casing and PVCseparator will come in with battery scrap.
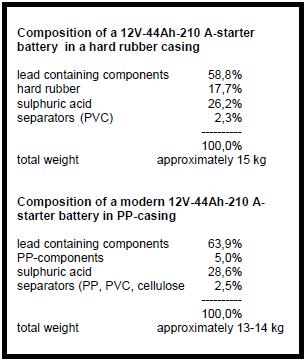
Depending on the type of battery, its size and design, the material composition of a starter battery varies. Table 1 compares an old type of battery in hard rubber casing with a modern type of battery. The lead-bearing components of a battery are:
The grids of old types of batteries have a higher Sb (antimony)-content (~4%) than the modern maintenance-free batteries (~2%), which instead add Ca(calcium) 0,5% to their grid alloy.
Recycling of Lead-acid Batteries
General Considerations
As already mentioned, lead-acid battery recycling has a long tradition, especially in industrialised countries. The battery and scrap trade takes back spent batteries free of charge or even pays the metal value. Because the metallic fraction of a battery consists largely of lead, metallurgical reprocessing of battery scrap was never a serious problem. Recently it has been rather the stricter environmental requirements that have caused problems for secondary lead smelters and made lead recycling less economically viable.
Lead recovery from spent accumulators can take two basic routes. Either the components of an accumulator like lead, plastics, acids, etc. are at first separated and then processed individually, or the acid is extracted first and the batteries are processed as a whole. In the first case, recycling materials are recovered from all components of a battery. In the second case, only lead is recovered (partially also residual battery acid), whereby organic components are consigned to energy recycling. In view of the high pollution control standards implemented in secondary lead smelters of industrialised countries, modern lead recycling does not pose a significant health hazard to the local population or the environment.
In developing countries spent lead batteries are recycled both in industrial facilities and by informal small enterprises. Industrial recycling smelters use both the grid metal and the lead-containing paste to produce secondary lead. The informal sector, in contrast, often only uses the metallic parts of old batteries (grids, terminals, bridges) to produce articles such as solders or weights for fishing nets. The other parts of the battery are simply dumped in the environment.
Even industrial recycling facilities in developing countries employ many manual techniques due to cheap labour. Batteries are often broken up, emptied, separated and charged to the furnaces by hand. The lead extracted is refined and cast into ingots manually. This creates a potential hazard for the workers, the surrounding population and the environment (soil, ground, water resources, etc.) in general.
Some secondary smelters also buy up pre-sorted battery fractions, e.g. grids and lead paste without casings and separators, in addition to complete batteries. The lead smelters thus save several processing stages and do not have to deal with casing and separator wastes. They are therefore willing to pay a higher price for the material supplied. This practice is very harmful in environmental terms. Through the dispersed pre-sorting activities, lead-containing residues and wastes arise in many places and it becomes impossible to control their proposal.
Metallurgical Aspects of Lead Recycling from Battery Scrap
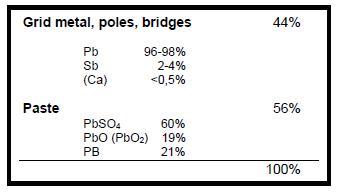
As described before, the lead bearing raw materials extracted from lead-acid battery scrap are:
- Pb(Sb) Metal from grids, terminals and bridges
- PbO (PbO2) lead oxides, part of the paste
- PbSO4 lead sulphate, part of the paste
While the first component needs only melting, the two other components have to be converted by chemical/metallurgical processes to obtain lead metal, which takes place in the furnace.
The first type of chemical reaction converts PbO (PbO2) into Pb Through a reduction process:
- 2 PbO + C -> 2 Pb + CO2
- PbO2 + C -> Pb + CO2
The second type converts PbSO4 into PbS, again through a reduction process:
- PbSO4 + 2 C -> PbS + CO2
Finally PbS is converted into Pb through the following reactions:
- PbS + Fe -> Pb + FeS
or
- PbS + 2 PbO -> 3 Pb +ü SO2
- PbS + PbO2 -> 2 Pb + So2
The above mentioned chemical reaction are sum reaction. That means that there are intermediate steps in between. The reactions take place in the melting furnace at high temperature (900-1200 °C) and need additives, which are . carbon (in the form of coal) and iron (in the form of iron swarf). Impurities are collected in the slag which requires for example soda ash as liquidifying and slag forming constituent. The product of the smelting operation is crude lead, which needs subsequent refining, and soda slag as residue. Since soda slag is water soluble and therefore hazardous when brought to landfills, modern lead recycling plants use silica slag (fayalite slag), which is water insoluble. It requires, however, a much higher furnace operation temperature of approximately 1400°C.
The refining of crude lead takes place in a refining kettle at temperatures between 400 and 550°C.
If only battery scrap is used for lead production, two subsequent refining steps are required:
- Removal of Cu which might have entered the melts through copper wires.
- Removal of antimony originated from the former grid metal to produce pure lead
While the removal of Cu is done in adding elementary sulphur, Sb can be removed by selective oxidation or by adding sodium nitrate (NaNO stirred and a dross formed. The impurities are now removed from the melt by skimming of the dross formed. It is obvious that the success of the refining has to be controlled by chemical analysis. The refined metal is cast into ingots for shipment, sale or further manufacturing.
Technical Steps in Battery Recycling
In developing countries lead-acid battery scrap is normally processed in rotary drum furnaces using liquid fuel as energy source. Lead bearing feed materials are either whole battery packs (grids and paste) where the separators have been removed or two separate fractions
- grid metal only and
- paste and other fines
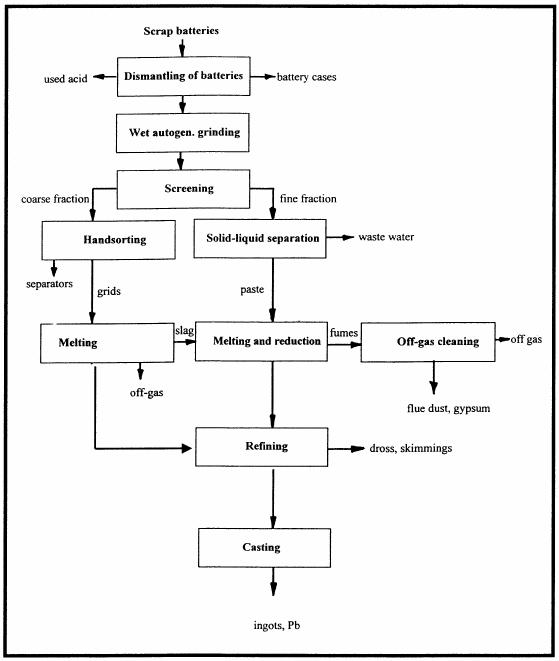
The flow sheet in Fig. 2 shows a semimechanised process option for small to medium scale battery recycling in developing countries. In this option grids and paste are separated and individually processed. Since the grids (2/5 of the total material) are already in the metal stage, metallurgical process of converting they do not need to go through the whole PbO/PbSO need to be molten at some 500°C (low temperature melt), refined and cast into ingots. Thus, energy and time are saved.
Dismantling of Battery Cases and Feed Preparation
Used batteries are emptied by hand and the acid is collected in plastic barrels. If the full barrels are kept motionless for some time, solid impurities will settle at the bottom of the barrels. This process of sedimentation may be assisted by adding some flocculent. The purified acid is then decanted and packed for sale. Possible customers for the recycled acid is the mining and metallurgical industry which uses acid in various leaching operations. The remaining battery sludge is neutralised with lime. After passing through a filter press the filter cake may be charged together with the fine fraction into the melting and reduction furnace.
In a next step the tops of the acid-free batteries are cut off by a guillotine shear and the grid packs are removed from the battery case. They are fed to a perforated grinding drum, which rotates in a water basin. By moving the feed in the drum an autogenous grinding process starts which separates the grids from the separators and, more important, the paste from the grids. At the same time the perforation of the drum acts as a sieve. The fines are separated and carried away by the water. A bit of lime added to the water neutralises the acidic solutions and prevents the drum from massive corrosion.
Instead of this labour intensive method whole batteries may be crushed in a hammer mill (Fig. 3) and fed to a grinding/ washing drum for separation.
In both cases, the slurry is pumped continuously or batchwise to sedimentation tanks, where the solids settle at the bottom. The clarified liquid is returned back to the grinding operation, while the sludge at the bottom of the tanks passes a filter press or is left to sun-dry. The filter or sun dried cake is the main feed for the melting and reduction operation which will produce almost pure lead.
The second fraction - the coarse material (basically grids and separators) - leaves the grinding drum at its lower end. Separators and grids are separated from each other by hand sorting using a slow moving transmission belt (Fig. 4). The metal fraction is the main feed for the low temperature melt producing a PbSb-alloy.
Empty battery cases and covers with the attached poles, bridges and remaining grid parts are charged to a wet hammer mill, where metal parts and remaining paste are separated from the plastic. The output of the mill passes a perforated drum, where solids and slurry are separated. While the solids (metal and plastic parts) are hand sorted, the slurry with the fines is added to the slurry obtained from the grinding drum. The solid metal parts supplement the feed of the low temperature melt.
The plastic residues of the dismantling operation either have to be dumped (in the case of PVC-separators) or can be used as fuel (PP, cellulose, hard rubber) in cement factories. In this case it is important that no lead remains in the plastic product.
Melting and Reduction Operation of Paste and Battery Fines
The filter or sun baked cake of paste is charged to a short rotary drum furnace (Fig. 5) where the charge is melted together with slag forming constituents (soda ash = Na2 CO3) and reaction additives (Fe-swarf, coal). The ratio of the feed materials Pb-fines : Fe-swarf : Soda ash : coal is approximately 10:2:1:0,5. The energy needed for the process is obtained from the burning of the coal within the furnace and by an additional burner running on heavy fuel oil, paraffin, diesel, waste engine oil, etc. To save energy and to achieve a higher furnace temperature the combustion air should be preheated.
Depending on the temperature and the amount of feed material in the furnace, the reaction time will be 2-3 h. Due to the difference in specific weight the molten lead produced settles at the bottom part of the furnace. When enough lead has accumulated, it is tapped into a mobile ladle and transported in liquid stage to the refining kettle.
With less PbO/PbS in the slag and more Pb-metal produced the viscosity of the slag increases. This hampers the separation of the small Pb-droplet from the slag. To overcome this problem either more soda ash has to be added or the temperature in the furnace must be increased. Both solutions have negative effects. While the first measure increases the amount of slag which finally needs to be discarded, the second measure leads to higher energy consumption and evaporation of lead into the off-gas.
It is more advisable to tap the lead before the optimum of recovery is achieved and to leave the remaining lead-rich slag in the furnace for a second or third cycle with new feed material.
After a number of production cycles the amount of slag in the furnace will be too large to continue the operation. By adding a bit more coal and fresh soda ash a slag wiith a low Pb content (9% Pb) can be achieved which is then tapped from the furnace together with the finally produced lead. While the lead metal is forwarded for refining the slag has to be dumped.
Off-gas and flue dust from the operation is sucked of and treated in the off-gas cleaning system.
Melting of Grids, Terminals and Bridges
The coarse fraction of the crushed battery scrap is fed to a crucible furnace, melting kettle or rotary drum furnace. By adding a bit of soda ash the charge is melted and stirred for some while. During this operation insoluble impurities will settle on top of the melt and join the soda ash slag, which is skimmed off at the end of the melting operation. Gases and flue dust from the process are soaked away and passed over to the gas cleaning system.
The melt is cast into ingots or transferred in liquid stage to the refining kettle.
Refining of Crude Lead
First, the lead tapped from the furnace has to be cleaned from residual oxides and slag. For that purpose a bit of pitch and saw dust is added. After stirring for a while the impurities settle at the surface and are skimmed off (Fig. 6).
Crude lead originating from battery scrap is normally alloyed with copper and antimony (with traces of Ca, Sn, As, Zn). In order to remove the unwanted elements two further refining operations have to be carried out.
By adding sulphur to the lead melt and after stirring for some time, a Pb/Cu2S-dross (and if present with minor parts of Zn, Sb, As) is formed and skimmed off. This de-copperisation step should be carried out at least two times to secure the refining result.
The de-copperised lead still contains a large amount of antimony (and maybe some Sn, As). All of these elements can be removed by oxidation. For that purpose air or oxygen-enriched air is blown into the melt and stirred. The different oxides formed settle at the surface and can be skimmed off. The oxidising process is completed when mainly lead oxide is formed.
Instead of oxidising the impurities by injecting air, sodium nitrate (NaNO3) can be added. Here again a dross containing the impurities (and lead) is formed, which is skimmed off afterwards. All refining by byproduced or residues should be processed to recover lead and other valuables components. The refining processes and the purity of the refined lead are monitored by chemical analysis. The off-gases of each of the processes are collected and fed into the central gas cleaning system of the plant.
Gas Cleaning System
Due to the lack of environmental legislation and monitoring, and due to lack of funds industrial operations in developing countries often have very poor emission control and off-gas cleaning systems. Because of the hazardous potential of the majority of the elements and compounds which are involved in lead smelting and refining (Pb, Sb, As, SO2, etc.), a certain gas cleaning standard must be achieved and should be compulsory.
Therefore, all fumes, gases and dusts which are generated during the different production steps should be collected and treated in a central gas cleaning system. A standard off-gas treatment system normally consists at least of a hot dust chamber and/or hot cyclone, a venturi washer and a wet scrubber (Fig. 7).
From the furnace the hot gases pass through a hot dust chamber and/or a hot cyclone where most of the coarse dust particles are separated from the gas stream. From there the off-gas feed into a wet gas cleaning system which consists of a venturi washer and a wet scrubber.
The task of the venturi washer is the collection of the fine dust particles. Water and off-gas is mixed under high turbulence and gas/water spray velocity, forming a fine slurry, which is pumped to a sedimentation tank.
The more or less dust free off-gas afterwards enters a wet scrubber. Here, the main task is the removal of the SO2- gas of the off-gas. By adding lime to the scrubber liquid, the SO2 in the off-gas will react with the lime water forming gypsum. The gypsum is insoluble in water and precipitates. Again, the fine slurry of the second scrubber is pumped into a sedimentation tank. The clean gas leaves the whole process via the main chimney. In the sedimentation tank small amounts of lime and flocculent neutralise the slurry and assist the sedimentation of the fines.
The sludge passes a filter press producing a filter cake. The extracted water is recirculated to the scrubbers while the filter cake is fed back into the melting and reduction furnace.
Environmental Considerations
As already mentioned, the potential health and environmental risk involved when processing battery scrap is very high.
Depending on the level of mechanisation and environmental standards, the following environmental hazards can arise:
- soil and groundwater contamination by acid spilled when batteries are emptied
- wind dispersal of lead dust if crushed battery scrap is stored without protection
- substantial atmospheric emissions (e.g. lead-containing dust, soot, SO2 chlorides, dioxins, etc.) when battery scrap is smelted due to:
- processing the entire battery including its organic parts (casing, PVC separators in older battery types)
- inadequate removel of gases and vapours during the smelting and refining process
- absent or inadeqaute flue gas treatment
- use of water-soluble soda slag without the corresponding landfill design that would prevent leaching and dust formation
- open storage of slag and ashes of the refining process
- open tipping of residues and wastes such as battery casings and PVC separators.
Workers, too, are exposed to raised levels of harmful substances in such facilities. This generates considerable health risks if appropriate precautionary measures are not taken (respiratory equipment, washing facilities, separate eating and resting rooms, regular examinations, etc.).
Further Information
- Presentation: recycling systems for PV-batteries, Andi Michel, Addis Ababa, October 2008
- Gate, GTZ: Vest, Heino: Fundamentals of the Recycling of Lead-Acid Batteries, 2002(279 kB)
- Pv-batteries, Silvia Schubert
References
- ↑ http://www.batterycouncil.org/
- ↑ GTZ waste management project
- ↑ Central African Batteries (Pvt) Ltd
https://energypedia.info/wiki/Recycling_of_Photovoltaic_(PV)_Batteries
https://energypedia.info/wiki/Recycling_of_Photovoltaic_(PV)_Batteries





ليست هناك تعليقات:
إرسال تعليق